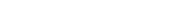Revolutionizing Battery Safety: Insights into Electrolyte Materials for All-Solid-State Batteries
lithium metal, potential dopant positions are illustrated as pink spheres. Colorful waves indicate gallium reduction and alloy formation post-lithium deposition. Credit: ACS Materials Letters (2024). DOI: 10.1021/acsmaterialslett.4c01237″ width=”800″ height=”450″/>
The Quest for Safer Battery Technology
A groundbreaking collaborative effort combining computational modeling and experimental research has been launched to explore how specific dopants can enhance the performance of solid electrolytes interacting with lithium metal electrodes. This advancement could pave the way for batteries that are not only safer but also more energy-efficient.
The Ubiquity of Lithium-Ion Batteries
Lithium-ion batteries are integral to our daily lives, powering everything from smartphones and laptops to electric vehicles (EVs). Recognizing their significance in contemporary technology, researchers continue to seek innovative ways to create batteries that prioritize safety and energy efficiency.
Recently published research by a team at the U.S. Department of Energy’s Argonne National Laboratory sheds light on solid electrolytes being evaluated for all-solid-state battery applications, offering promising strategies toward achieving these goals.
This novel research appears in the journal ACS Materials Letters.
What Makes Solid Electrolytes Unique?
Electrolytes function as vital membranes that facilitate the movement of electrical charges carried by lithium ions between a battery’s positive and negative terminals. Unlike traditional liquid electrolytes found in conventional lithium-ion batteries, all-solid-state designs employ solid counterparts—a shift identified as crucial for developing lightweight, high-density energy storage solutions that last longer and maintain higher safety standards.
Solid electrolytes boast significant advantages; they lack volatility or flammability compared to their liquid counterparts, contributing to safer battery operations while exhibiting improved compatibility with lithium metal electrodes due to reduced reactivity.
The Energy Advantages of Lithium Metal
Lithium metal offers superior energy density compared to graphene-based alternatives like graphite because every atom can participate in charge/discharge processes within a battery system—allowing it to store significantly more energy overall.
Dopant Innovations Within Lithium Lanthanum Zirconium Garnet (LLZO)
Lithium lanthanum zirconium garnet (LLZO) emerges as a frontrunner among solid electrolyte materials due largely its impressive strength, durability, and noteworthy ionic conductivity—the property enabling efficient ion transport during charging cycles.
Researchers have initiated experiments by introducing trace amounts of elements such as aluminum or gallium into LLZO structures—a technique referred to as doping—which enhances ionic conductivity much like adding flavorful spices elevates an ordinary dish into something exceptional.
The Dual Nature of Doping Effects
Additions like aluminum or gallium maintain LLZO’s symmetric structure while creating vacancy sites that expedite ion migration from electrodes—thereby boosting conductivity levels significantly. However, such enhancements may inadvertently increase LLZO’s reactivity with metallic lithium; this could diminish cycle life expectancy across various applications.
Catalyzing Findings on Gallium-Doped LLZO
This investigation examined interactions between aluminum- or gallium-doped LLZO when exposed directly alongside metallic lithium surfaces using advanced computational methods complemented by experimental validation techniques.
- The findings revealed an increased propensity for gallium ions in doped structures relocating away from surrounding lattices after contact with metallic lithiums forming alloyed compounds).
- This not only accelerates depletion but potentially alters structural integrity subsequently diminishing overall conductivity metrics observed during testing protocols).
Dynamics Between Aluminum & Galliam:
Since Aluminum-Doped variants prominently survive compositional reshaping paths introduced via interactions revealing comparatively robust setup sustaining reliable characteristics imparted through stable-distance reactions cumulatively establishing trustworthy frameworks conducive toward future product developments!
Paving Paths Towards Separtation Between Reactivity Displaying Compounding Effects!
If hypotheses remain upheld thus paving avenues unlocking limits separating effective conductivities revealing stability constraints upon allocations necessitating innovation encapsulation proactively assisting inquiries surrounding achieving harmonized configurations amidst various material/process complexities presented sustainably improving prospects enhancing durability whilst anticipating demands imposed aligning upcoming technological transformations anticipated hence signified widespread integration across industries reliant correlating demands persisting growing globally!< / P >
Revolutionizing Battery Technology: Insights into Solid-State Electrolytes
The researchers employed advanced computer simulations, specifically density functional theory, to examine the behavior of atoms and electrons within different materials. This approach enabled them to forecast the stability of several dopants and predict their interactions with various compounds.
Challenges in Analyzing Electrochemical Interfaces
Gaining insight into the solid electrolyte-electrode interface presents considerable challenges for scientists, particularly during active electrochemical reactions within batteries. As noted by Tepavcevic, these interfaces are often “entombed” and escape detection by conventional experimental methods.
Innovative Experimental Techniques
The team utilized X-ray photoelectron spectroscopy (XPS) to investigate alterations in the surface chemistry of lithium lanthanum zirconium oxide (LLZO). Additionally, they employed electrochemical impedance spectroscopy (EIS) to scrutinize lithium ion mobility both in electrolytes and at the electrolyte-electrode junction.
To gain further insights regarding atomic structures within materials, researchers harnessed neutron diffraction. This technique confirmed that while gallium’s stability diminished and reactivity increased upon contact with lithium, aluminum maintained its stability throughout the interactions.
Collaboration Enhances Research Outcomes
This study benefited significantly from partnerships with multiple institutions. The University of California, Santa Barbara was instrumental in supplying high-grade LLZO samples. Furthermore, neutron diffraction experiments took place at established facilities at Germany’s Heinz Maier-Leibnitz Zentrum as well as at the Nuclear Physics Institute associated with the Czech Academy of Sciences.
“The collaboration between U.S. and German teams has been essential for our research,” conveyed Zapol. “These discoveries pave new pathways toward developing safer and more efficient solid-state battery technology on a global scale.”
Acknowledgments from Collaborative Authors
The team comprised notable contributors such as Matthew Klenk, Michael Counihan, Zachary Hood, Yisi Zhu, Justin Connell from Argonne National Laboratory; along with Neelima Paul and Ralph Gilles from Heinz Maier-Leibnitz Zentrum; Charles Hervoches from Nuclear Physics Institute; and Jeff Sakamoto representing UC Santa Barbara.
Citation for Further Reading:
Klenk et al., “Comparative Analysis of Reactivity of Al- versus Ga-Doped Garnet Solid State Electrolyte Interface with Lithium Metal,” published in ACS Materials Letters (2024). DOI: 10.1021/acsmaterialslett.4c01237
Innovations in Battery Safety: New Discoveries for Enhanced Technology
Introduction to Battery Safety Challenges
As technology continuously evolves, the demand for safer and more efficient batteries has reached unprecedented levels. With the rise of electric vehicles and renewable energy solutions, ensuring battery safety is paramount. Recent advancements offer significant insights into enhancing battery durability and minimizing risks associated with battery malfunctions.
Unveiling Critical Insights
Recent research has shed light on key factors influencing battery safety. Scientists have identified specific components that contribute to thermal stability within batteries. This discovery could pave the way for developing next-generation batteries that resist overheating and other associated hazards.
In a groundbreaking study, researchers analyzed various materials commonly used in battery production. Their findings revealed that certain compounds exhibit improved resistance to thermal runaway reactions—an essential element in preventing catastrophic failures during operation.
Statistical Spotlight: The Importance of Innovation
According to industry analyses by 2023, electric vehicle sales surged by over 25%, underscoring an urgent need for safer energy storage solutions. Furthermore, reports indicate that around 30% of recalled vehicles were linked to faulty battery performance in previous years, which highlights the increasing necessity for enhanced safety measures within this sector.
Alternatives That Change the Game
Innovations aren’t limited to just one material or design change; instead, they encompass a holistic approach toward producing safer batteries. For instance, researchers are exploring solid-state batteries as a viable alternative to traditional lithium-ion designs. Solid-state technologies promise increased efficiency while significantly reducing risks related to leaks or fires commonly seen with current variants.
Moreover, advancements like flame-retardant electrolytes are gaining traction. These innovative elements can be integrated into existing systems without major redesigns while offering higher safety standards under extreme conditions—potentially lowering failure rates considerably.
Conclusion: A Safer Future on the Horizon
The path toward creating safer batteries is laden with challenges but also brimming with opportunity as research unravels crucial insights needed for future development. By implementing innovative materials and designs today, we can expect substantial improvements in both efficiency and user confidence tomorrow—transforming how we perceive energy storage systems across industries from automotive to consumer electronics.
With ongoing studies suggesting promising directions forward coupled with rising market demands, it becomes increasingly clear that investing time and resources into these innovations is essential for fostering a sustainable energy future.