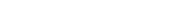hydrogen peroxide from the atmosphere for industrial purposes” title=”Illustration of Zn-Air battery operation and dye decomposition. Source: AJB lab, IISc” width=”800″ height=”450″/>
Innovative Production of Hydrogen Peroxide Using Zinc-Air Batteries
Hydrogen peroxide (H2O2) serves various vital roles, including its use as a bleaching agent, disinfectant, and an oxidizer. Nevertheless, the traditional methods for producing H2O2 at an industrial scale are costly and energy-intensive due to reliance on rare metal catalysts.
Onsite Development of Hydrogen Peroxide
A team at the Indian Institute of Science (IISc) has conceptualized a groundbreaking technique for onsite hydrogen peroxide production that simultaneously tackles the degradation of hazardous dyes in industrial effluents.
The researchers employed a zinc-air battery approach where H2O2 is generated through an oxygen reduction reaction. “Zinc is not only plentiful but also economically viable in India,” highlights Aninda J Bhattacharyya, Professor at both the Interdisciplinary Center for Energy Research (ICER) and Solid State and Structural Chemistry Unit (SSCU), who contributed significantly to a study published in Small Methods.
Understanding Metal-Air Battery Mechanism
A metal-air battery operates by utilizing zinc as its anode (negative electrode) while using ambient air as its cathode (positive electrode). During discharge—when power is released—the process involves reducing atmospheric oxygen at the cathode to yield H2O2.
The formation of H2O2 occurs through one pathway during electrochemical oxygen reduction. “The key here lies in managing how much we reduce oxygen; uncontrolled reactions will just produce water instead,” Bhattacharyya points out.
Catalyst Innovations for Enhanced Selectivity
Specific catalysts facilitate this control over reactions. “Our approach uses a carbon-based catalyst devoid of metals,” adds Asutosh Behera, primary author and Ph.D. candidate at SSCU. These lower-cost catalysts typically route reactions towards water generation with lesser selectivity towards forming hydrogen peroxide.
However, by modifying these catalysts chemically—like incorporating functional groups containing oxygen—we can effectively increase their propensity to favor H2O2 production instead.
A Groundbreaking Application
This novel technique represents a direct method to produce hydrogen peroxide from within a battery system itself without requiring additional processes or equipment. “By adjusting voltage specifically within our design, we can ensure that only H2O2 formation occurs,” explains Bhattacharyya further.
Dye Detection Tied to Hydrogen Peroxide Generation
An intriguing aspect involves detecting generated H₂O₂ since it is colorless; thus introduction of toxic dyes—which are prevalent pollutants from textile manufacturing—serves as an effective indicator. Upon synthesis with those dyes, produced hydrogen peroxide instigates degradation via alteration in color signaling its presence.
“This generated hydrogen peroxide subsequently breaks down into various reactive radicals like hydroxide or superoxide radicals which efficiently lead towards decomposing textile dyes,” notes Behera.< p>{}
Tackling Challenges Ahead
“Despite some fundamental issues related primarily about handling three phases present—involving solid zinc phase along with liquid electrolyte plus gas air—it presents greater complexity than most conventional batteries working on two phases,” cautions Bhattacharyya:
Sustainability Potential & Future Prospects
- The prospects appear optimistic concerning scalability along with alternative practical applications such as electricity generation even within remote areas.” indicates Bhattacharya confidently.“ This methodology stands out owing notably being sustainable alongside low-cost while maintaining impressive energy efficiency” he concludes…
Source:Indian Institute Of Science
Citation:
Innovative Methodologies Using Batteries To Formulate Hydrogen Peroxide From Atmospheric Components For Industrial Applications (
March 04 2025).
Portions may not be reproduced without written consents more than fair use applies.
Information offered served solely aims educational clarity�