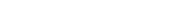sulfur batteries with lavender oil” title=”a) A diagram depicting the creation of sulfur-carbon composite material and b) a schematic of the inverse vulcanization process involving elemental sulfur and linalool. Source: Small (2024). DOI: 10.1002/smll.202407300″ width=”800″ height=”471″/>
Revolutionizing Energy Storage: The Role of Lavender Oil
A groundbreaking discovery by researchers at the Max Planck Institute of Colloids and Interfaces suggests that lavender oil, particularly its primary component linalool, could significantly enhance the performance and longevity of sodium-sulfur batteries. These improvements could play a vital role in efficiently storing electricity generated from renewable energy sources.
Storing Renewable Energy Effectively
The challenge of effectively storing excess electricity from renewable generation—such as wind and solar—is crucial in today’s energy landscape. Sodium-sulfur batteries stand out as promising candidates for stationary storage due to their abundance compared to lithium-based alternatives.
The Advantages and Limitations of Sodium-Sulfur Batteries
Sodium-sulfur batteries are built using materials that are more readily available than lithium or cobalt, whose extraction can lead to significant environmental degradation along with social issues at mining sites. Despite these advantages, current sodium-sulfur technology faces limitations regarding energy density relative to weight when compared to lithium counterparts.
Extending Battery Life with Natural Solutions
According to a recent study published in the journal Small, integrating linalool derived from lavender oil into battery design is showing promise in overcoming one major limitation—rapid capacity loss due to polysulfide migration during charge cycles.
Breaking Down Sulfur Shuttling Issues
Sodium-sulfur batteries typically face diminished efficiency after multiple charge-discharge cycles primarily due to sulfur shuttling processes where polysulfides migrate from the cathode to react adversely at the anode. Evgeny Senokos leads efforts at Max Planck aiming for innovative solutions by encapsulating these problematic substances within robust carbon structures.
Developing Advanced Nanomaterials
The research team has succeeded in creating a stable nanomaterial composed of both linalool and sulfur which forms ultra-narrow pores roughly 100,000 times thinner than human hair—effectively trapping polysulfides while allowing sodium ions necessary for operation through their network during charging cycles.
Remarkable Performance Metrics Achieved
This approach has yielded impressive results; battery cells demonstrated over 80% retention of initial charging capacity even after undergoing 1,500 full charge-discharge cycles—a notable enhancement over traditional designs. Furthermore, this novel cathode material boasts an outstanding capacity exceeding 600 mAh/g due largely to more effective usage of fixed sulfur within its structured confines.
A New Dawn for Sustainable Energy Solutions
“By examining nature’s offerings creatively,” remarks Giusto , ”we’re discovering innovative approaches toward addressing numerous challenges related to our transition into sustainable energy.” He expresses optimism about moving this technology beyond laboratory confines towards real-world applications soon.
Citation:
Evgeny Senokos et al., ”Sustainable Sulfur‐Carbon Hybrids for Efficient Sulfur Redox Conversions in Nanoconfined Spaces,” Small (2024). DOI: 10.1002/smll.202407300
Date:
The text summarized here was made available on January 27th, 2025.